CMS initiates the NCD process by opening the NCD. This is announced to the public by posting a tracking sheet on the CMS coverage website. NCD reviews pertain to reviews of a particular item or service to determine whether they meet the statutory requirements. Development of a complete, formal request for an NCD can be initiated either by an outside party or internally by CMS staff.
NCDs are made through an evidence-based process with opportunities for public notice and comment participation. NCD requests are accepted on a rolling basis. If CMS has a large volume of NCD requests for simultaneous review, requests are prioritized based on the magnitude of the potential impact on the Medicare program and its beneficiaries and staffing resources. For more information, please see: §1862(l) of the Act and 78 FR 48164, August 7, 2013.
Important elements of a complete, formal NCD request include:
For more in-depth information refer to instructions on how to request an NCD .
An NCD reconsideration request generally follows the same process as requesting a new NCD.
CMS may consider accepting a request to revise an existing NCD at any time, but only if the requester presents documentation that meets one of the following criteria:
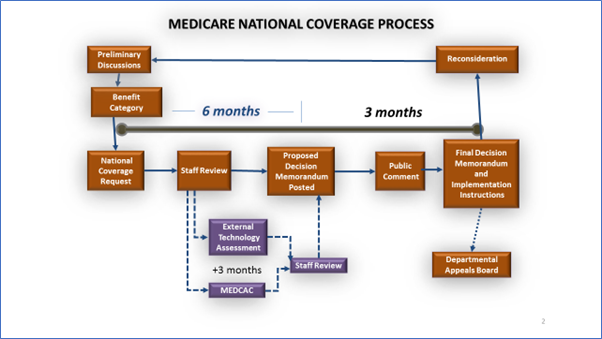
The NCD process generally takes nine to twelve months. The time-frame does not begin until CMS formally accepts an NCD request and informs the public that an item or service is under consideration by posting a notice (i.e., a tracking sheet) on the CMS coverage webpage. Though not required by law, CMS customarily also invites public comment on the opening of the NCD. In general, six months after CMS posts the tracking sheet, a proposed NCD must be published with a statutorily required 30-day public comment period. If an NCD analysis includes an external technology assessment or Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) meeting, the proposed NCD must be published within nine months after posting the tracking sheet, rather than six. A final decision must be published within 60 days after the public comment period closes and is effective upon posting the final decision on the CMS Coverage website. To view the National Coverage Analyses (NCAs) and Coding Analyses for Labs (CALs) currently available for public comment, visit the CMS Medicare Coverage Database and select “National Coverage NCA/CAL Open for Public Comment” from the drop-down menu.

Technology Assessments are systematic reviews of evidence, conducted and coordinated by CMS staff to review relevant evidence and inform a determination if the item or service is reasonable and necessary. To minimize bias, systematic reviews emphasize a comprehensive search of all potentially relevant medical and scientific articles and use explicit, reproducible criteria in the selection of articles for review. Data is summarized and the evidence is appraised to assess its validity (how credible it is), clinical relevance (its applicability in real health care settings), and weight (magnitude of effect).
CMS may determine that an external technology assessment is necessary for cases where there is not an extensive body of evidence to review, there is disagreement among experts, or the review requires unique technical or clinical expertise not available within CMS staff at the time of the review. For more information about how CMS determines whether a Technology Assessment is necessary refer to factors CMS considers when commissioning an external Technology Assessment.
CED is a paradigm whereby Medicare covers items and services on the condition that they are furnished in the context of approved clinical studies or with the collection of additional clinical data. In making coverage decisions involving CED, CMS decides after a formal review of the medical literature to cover an item or service only in the context of an approved clinical study or when additional clinical data are collected to assess the appropriateness of an item or service for use with a particular beneficiary.
Earlier CED decisions were made under section 1862(a)(1)(A) of the Act. More recent NCDs have tended to rely on section 1862(a)(1)(E) of the Act, in which CED is used to support clinical research. Section 1862(a)(1)(E) of the Act, provides, in pertinent part, that:
Section 1142 of the Act instructs the Agency for Healthcare Research and Quality (AHRQ) to conduct and support research on outcomes, effectiveness, and appropriateness of services and procedures to identify the most effective and appropriate means to prevent, diagnose, treat, and manage diseases, disorders, and other health conditions. Section 1142(b)(3) of the Act requires that the Secretary assure that AHRQ research priorities appropriately reflect the needs and priorities of the Medicare program.
For more information refer to:
The MEDCAC was established to provide independent guidance and expert advice to CMS on specific clinical topics. The MEDCAC is used to supplement CMS' internal expertise and to allow an unbiased and current deliberation of "state of the art" technology and science. The MEDCAC reviews and evaluates medical literature, reviews technology assessments, reviews public testimony, and examines data and information on the benefits, harms, and appropriateness of medical items and services that are covered under Medicare or that may be eligible for coverage under Medicare. The MEDCAC judges the strength of the available evidence and makes recommendations to CMS based on that evidence. Additional information on the MEDCAC, including the factors CMS considers in determining whether to refer topics to the MEDCAC, can be found on the Medicare Evidence Development & Coverage Advisory Committee webpage.
IMPORTANT: This information is only intended as a general summary and is not intended to grant rights or impose obligations nor is it intended to establish or change any substantive legal standards established under statutory or regulatory authority. This site contains references and links to certain statutes, regulations, and other policy materials, but it is not intended to be an all-inclusive listing or take the place of applicable statutory law or regulations. We encourage readers to review the specific statutes, regulations, and other interpretive materials for a full and accurate statement of their contents.